Publications, documents, patents, presentations, etc.
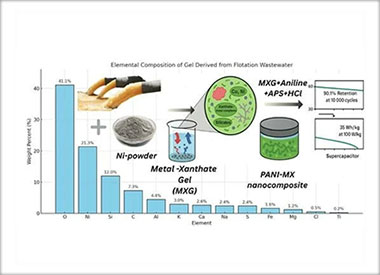
Utilization of Flotation Wastewater for Metal Xanthate Gel Synthesis and Its Role in Polyaniline-Based Supercapacitor Electrode Fabrication
The aim of this study is to explore the feasibility of using flotation wastewater from copper–porphyry ore processing to synthesize a gel that serves as a precursor for a polymer nanocomposite used in supercapacitor electrode fabrication. These wastewaters—characterized by high acidity and elevated concentrations of metal cations (Cu, Ni, Zn, Fe), sulfates, and organic reagents such as xanthates, oil (20 g/t ore), flotation frother (methyl isobutyl carbinol), and pyrite depressant (CaO, 500–1000 g/t), along with residues from molybdenum flotation (sulfuric acid, sodium hydrosulfide, and kerosene)—are byproducts of copper–porphyry gold-bearing ore beneficiation. The reduction of Ni powder in the wastewater induces the degradation and formation of a gel that captures both residual metal ions and organic compounds—particularly xanthates—which play a crucial role in the subsequent steps. The resulting gel is incorporated during the oxidative polymerization of aniline, forming a nanocomposite with a polyaniline matrix and embedded xanthate-based compounds. An asymmetric supercapacitor was assembled using the synthesized material as the cathodic electrode. Electrochemical tests revealed remarkable capacitance and cycling stability, demonstrating the potential of this novel approach both for the valorization of industrial waste streams and for enhancing the performance of energy storage devices.

Газ-дифузионен електрод с еднослойна структура за презареждаеми метал хидрид-въздух батерии
Газ-дифузионен електрод с еднослойна структура за презареждаеми метал хидрид-въздух батерии
Рег. № 67713 B1
Заявка № 113620
Дата на заявяване: 18 ноември 2022 г.
Публикувано за издаване: 15 април 2025 г.
Срок на действие: 18 ноември 2024 г.
Изобретатели: В. Н. Терзиев, Б. И. Абрашев, М. Г. Пандев, К. М. Петров, Д. Р. Леви.
Притежател: Институт по електрохимия и енергийни системи "Акад. Евгени Будевски"
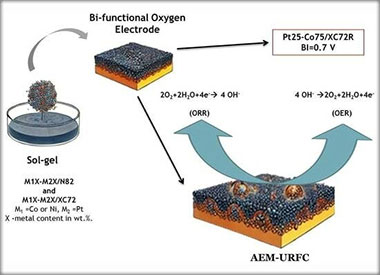
Sol–Gel-Synthesized Pt, Ni and Co-Based Electrocatalyst Effects of the Support Type, Characterization, and Possible Application in AEM-URFC
This study explores the synthesis and characterization of platinum (Pt), nickel (Ni), and cobalt (Co)-based electrocatalysts using the sol–gel method. The focus is on the effect of different support materials on the catalytic performance in alkaline media. The sol–gel technique enables the production of highly uniform electrocatalysts, supported on carbon-based substrates, metal oxides, and conductive polymers. Various characterization techniques, including X-ray diffraction (XRD) and scanning electron microscopy (SEM), were used to analyze the structure of the synthesized materials, while their electrochemical properties, which are relevant to their application in unitized regenerative fuel cells (URFCs), were investigated using cyclic voltammetry (CV) and linear sweep voltammetry (LSV). This hydrogen energy-converting device integrates water electrolyzers and fuel cells into a single system, reducing weight, volume, and cost. However, their performance is constrained by the electrocatalyst’s oxygen bifunctional activity. To improve URFC efficiency, an ideal electrocatalyst should exhibit high oxygen evolution (OER) and oxygen reduction (ORR) activity with a low bifunctionality index (BI). The present study evaluated the prepared electrocatalysts in an alkaline medium, finding that Pt25-Co75/XC72R and Pt75-Co25/N82 demonstrated promising bifunctional activity. The results suggest that these electrocatalysts are well-suited for both electrolysis and fuel cell operation in anion exchange membrane-unitized regenerative fuel cells (AEM-URFCs), contributing to improved round-trip efficiency.
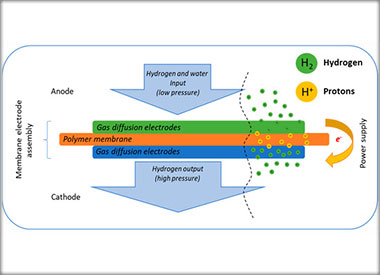
Electrochemical Hydrogen Pump/Compressor in Single-and Double-Stage Regime
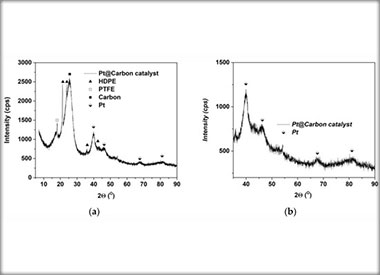
This study presents the integration and evaluation of commercially available gas diffusion electrodes (GDEs), specifically designed for high-temperature polymer electrolyte membrane fuel cells (HT-PEMFCs) within membrane electrode assemblies (MEA) for electrochemical hydrogen pump/compressor applications (EHP/C). Using Nafion 117 as a solid polymer electrolyte, the MEAs were analyzed for cell efficiency, hydrogen evolution, and hydrogen oxidation reactions (HER and HOR) under differential pressure up to 16 bar and a temperature ranging from 20 °C to 60 °C. Key properties of the GDEs, such as electrode thickness and conductivity, were investigated. The catalytic layer was characterized via XRD and EDX analyses to assess its surface and bulk composition. Additionally, the effects of increasing MEA’s geometric size (from 1 cm2 to 5 cm2) and hydrogen crossover phenomena on the efficiency were examined in a single-cell setup. Electrochemical performance tests conducted in a single electrochemical hydrogen pump/compressor cell under hydrogen flow rates from 36.6 Ml·min⁻1·cm⁻2 to 51.3 mL·min⁻1 cm⁻2 at atmospheric pressure provided insights into the optimal operational parameters. For a double-stage application, the MEAs demonstrated enhanced current densities, achieving up to 0.6 A·cm⁻2 at room temperature with further increases to 1 A·cm⁻2 at elevated temperatures. These results corroborated the single-cell data, highlighting potential improvements in system efficiency and a reduction in adverse effects. The work underscores the potential of HT-PEMFC-based GDEs for the integration of MEAs applicable to advanced hydrogen compression technologies.
Catalytic Activity of Pt/Pd Mono-and Bimetallic Catalysts in Electrochemical Hydrogen Pump/Compressor
In this study, mono- and bimetallic platinum (Pt), palladium (Pd) and Pt-Pd nanoparticles were synthesized using the wet sol–gel method, employing a carbon-based XC72R as catalytic carrier. The overall metal content was set at 40 wt.% at varying Pt:Pd ratios. Characterization of the morphology and surface structure was conducted through scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX), Brunauer–Emmett–Teller (BET) and X-ray diffraction (XRD) analyses. The electrochemical performance and catalytic activity against the hydrogen evolution reaction (HER) were assessed in a three-electrode cell for screening purposes, as well as in a prototype cell of an electrochemical hydrogen pump/compressor (EHP/C) where the catalysts served as cathodes, while the anode was Pt/XC72 40% wt. with 0.38 mgPt·cm−2 within a membrane electrode assembly (MEA) with a 180 µm thick Nafion 117 proton-conductive membrane. The results obtained indicated superior catalytic activity of the bimetallic catalysts in comparison to the pure metal samples. Further electrochemical tests in an EHP/C cell at varying differential pressures in the range of 0–3 bar revealed stable behavior and high current density, reaching approximately 0.7 A cm−2 at 60 °C. The accelerated durability tests performed demonstrated excellent stability of the synthesized composite catalysts. These findings underscore the potential of Pt-Pd nanoparticles as efficient catalysts with sustainable performance for electrochemical hydrogen pumping/compressing applications.
